You are here: English > Cold Chain > Cold chain pharmaceuticals
If we take a closer look to the types of pharmaceutical products or healthcare products, we see that several product groups are formed depending on the temperature barrier in which the products must be kept in order to guaranty the quality of the product. The most common known product groups are chilled products, ambient products and frozen products which each have their own specific temperature barriers. The most common temperature barrier in time-temperature sensitive chilled products is (+2°C to +8°C) temperature barrier. This means that during the total cold chain, from the pharmaceutical producer to the consumer, the temperature may not sink below (+2°C) or rise above (+8°C). If we look at ambient pharmaceuticals or healthcare products the most common temperature barriers are (+15°C to +25°C) or (+0°C to (+25°C). This also means that during the total cold chain, from producer to consumer, the temperature may not sink below the minimal barrier or rise above the maximal barrier. This Ambient product group is only recently obliged to certain temperature barriers due to new laws and rules.
Cold chain of temperature sensitive pharmaceuticals and healthcare products
A cold chain, also called a temperature controlled supply chain, is a chain in which time-temperature sensitive products are transported or distributed from a starting point, the sender of the products to an end point, the end-user of the products. A cold chain is build-up by multiple actors who actually make the cold chain. These actors can be:
- The producer of the time-temperature sensitive products.
- The warehouses where the products are stored.
- Transport companies who transport the products.
- Freight forwarders who handle the products.
- Distribution centers and sales locations
All these actors create the cold chain and determine its success or failure.
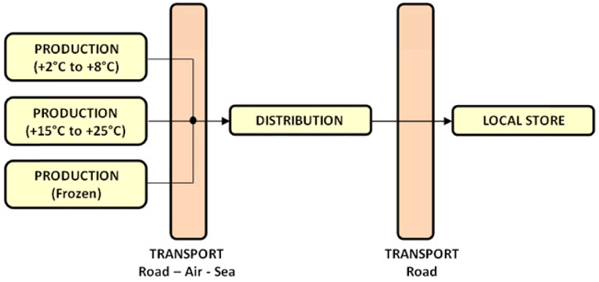
Above, a cold chain is shown with the different types of pharmaceutical products or healthcare products. The drawing shows that a producer of time-temperature sensitive products can produce multiple types of products such as chilled, ambient and frozen, which need to be handled by all the actors in the cold chain. The question which needs to be asked is if the actors in the cold chain are equipped with the required equipment to handle these products where the minimal and maximal temperature barriers of the different products are preserved. They therefore need to implement temperature controlled environments (active cooling systems) or packaging systems (passive cooling systems), or a combination of both, so the required temperatures can be offered.
With other words, the big question in a cold chain is if the cold chain is closed meaning that in the total cold chain no temperature excursions appear, which we call HCCP’s or “Hazardous Critical Control Points”, where the temperature of the products can drop below the minimal product temperature barrier or rise over the maximal temperature barrier.
Multiple temperature cold chains
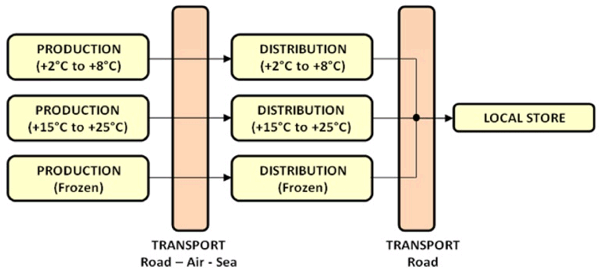
The above drawing shows a cold chain in which three pharmaceutical producing companies are producing three different types of time-temperature sensitive pharmaceutical products and where three different distribution channels are created meaning that different transport and warehousing companies handle each specific product. The challenge becomes even greater when all the different time-temperature sensitive products are handled by a single distribution channel, as shown below, meaning that one warehousing or transport company handles all the different products. Than the warehouse or transport company needs to be equipped with the required equipment to handle all these products within each separate temperature range. This is not an easy task in which mostly a combination of active and passive cooling systems are used.